
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
BP/USP/EP
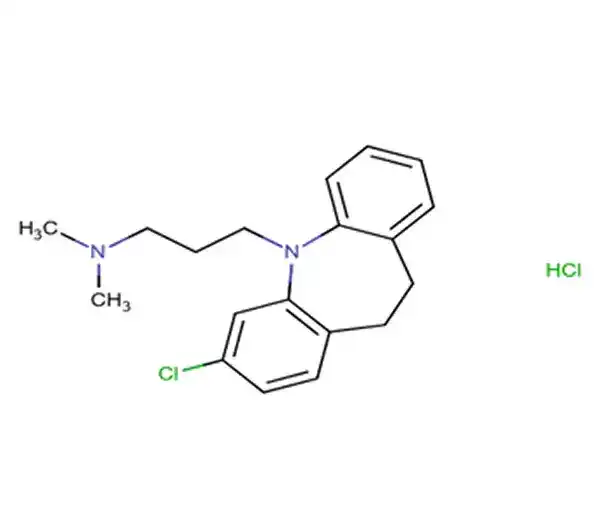
C37H67NO13
(3R,4S,5S,6R,7R,9R,11R,12R,13S,14R)-6-[(2S,3R,4S,6R)-4-(DIMETHYLAMINO)-3-HYDROXY-6-METHYLOXAN-2-YL]OXY-14-ETHYL-7,12,13-TRIHYDROXY-4-[(2R,4R,5S,6S)-5-HYDROXY-4-METHOXY-4,6-DIMETHYLOXAN-2-YL]OXY-3,5,7,9,11,13-HEXAMETHYL-1-OXACYCLOTETRADECANE-2,10-DIONE
114-07-8
733.94 g/mol
Macrolide antibiotic
| Appearance | White to off-white powder or lyophilized solid |
|---|---|
| Solubility | Practically insoluble in water; soluble in ethanol, methanol |
| Melting point | 135°C and 140°C |
| pH | 6.8 to 7.2 |
Erythromycin is a macrolide antibiotic used to treat a wide range of bacterial infections. It works by inhibiting bacterial protein synthesis, thereby preventing bacterial growth. It is effective against respiratory tract infections, skin infections, and sexually transmitted infections, among others. Erythromycin is available as a powder for formulation or as various oral and injectable dosage forms.
Erythromycin binds to the 50S ribosomal subunit of susceptible bacteria, inhibiting translocation of peptides during protein synthesis. This action prevents bacterial growth and replication, making it bacteriostatic at low doses and bactericidal at higher doses.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Erythromycin treats bacterial infections like respiratory tract infections, skin infections, and certain sexually transmitted infections.
Yes, with proper regulatory approvals and compliance with export laws.
Its compliance with BP/USP/EP standards and GMP-certified manufacturing makes it export-ready.
Contact Salius Pharma with quantity, destination, and documentation requirements to receive a formal quotation and export guidance.
Looking to source Erythromycin or other high-quality pharmaceutical products? We’re here to help. Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support, our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.