
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
BP/USP/EP
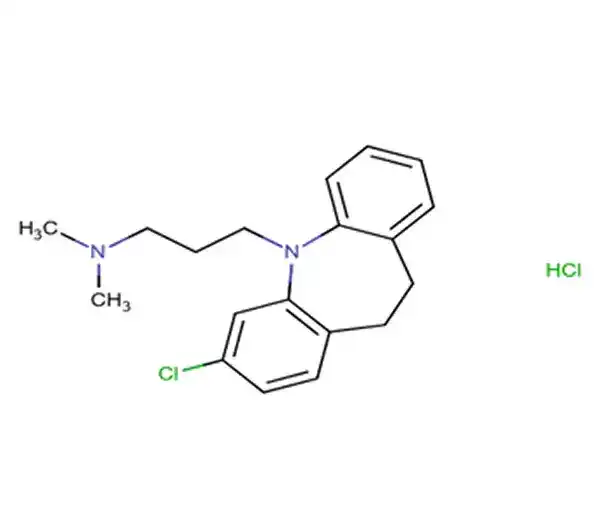
C32H39NO4⋅HCl
2-(4-{1-hydroxy-4-[4-(hydroxydiphenylmethyl)piperidin-1-yl]butyl}phenyl)-2-methylpropanoic acid hydrochloride
153439-40-8
538.13 g/mol
Second-generation antihistamine
| Appearance | White to off-white powder or lyophilized solid |
|---|---|
| Solubility | Slightly soluble in water, freely soluble in methanol and ethanol |
| Melting point | 198–202 °C |
| pH | 5.5–7.0 (1% aqueous solution) |
Fexofenadine HCl is a non-sedating, second-generation antihistamine used for the relief of allergic rhinitis, chronic urticaria, and other histamine-mediated conditions. It is available as an API for formulation into tablets, capsules, and suspensions.
Fexofenadine selectively blocks peripheral H1 histamine receptors, preventing the action of histamine responsible for allergic symptoms such as sneezing, runny nose, and itching. Being non-sedating, it does not cross the blood-brain barrier significantly, reducing drowsiness.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Relieves allergic symptoms such as sneezing, itching, and runny nose.
Protect from moisture, heat, and light to maintain stability.
Its compliance with BP/USP/EP standards and GMP-certified manufacturing makes it export-ready.
Contact Salius Pharma with quantity, destination, and documentation requirements to receive a formal quotation and export guidance.
Looking to source Fexofenadine Hydrochloride or other high-quality pharmaceutical products? We’re here to help. Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support, our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.