
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
BP/EP
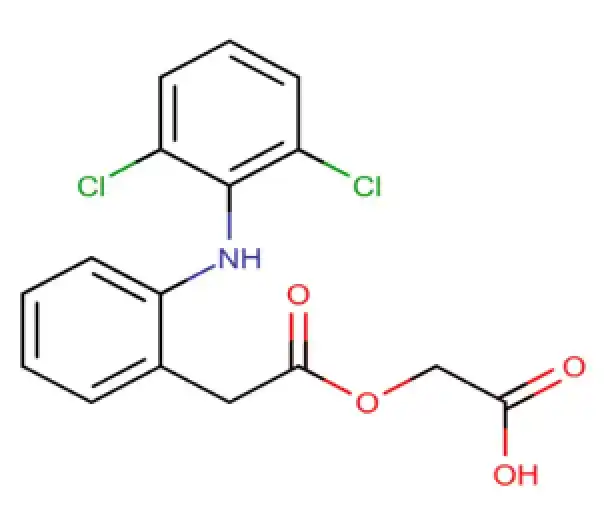
C16H13Cl2NO4
2-[(2-{2-[(2,6-dichlorophenyl)amino]phenyl}acetyl)oxy]acetic acid
89796-99-6
354.18 g/mol
Anti-inflammatory
Dichlorobenzenes
NSAIDs
| Appearance | White to almost white crystalline powder |
|---|---|
| Solubility | Practically insoluble in water; freely soluble in ethanol & acetone; soluble in methanol & chloroform |
| Melting point | 149–153 °C |
| pH | 6.0 – 7.5 |
Aceclofenac is an oral non-steroidal anti-inflammatory drug (NSAID) with marked anti-inflammatory and analgesic properties used to treat osteoarthritis, rheumatoid arthritis and ankylosing spondylitis. Aceclofenac potently inhibits the cyclo-oxygenase enzyme (COX) that is involved in the synthesis of prostaglandins, which are inflammatory mediators that cause pain, swelling, inflammation, and fever.
Aceclofenac is a NSAID that inhibits both isoforms of COX enzyme, a key enzyme involved in the inflammatory cascade. COX-1 enzyme is a constitutive enzyme involved in prostacyclin production and protective functions of gastric mucosa whereas COX-2 is an inducible enzyme involved in the production of inflammatory mediators in response to inflammatory stimuli.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO-GMP, ISO 9001:2015, FDA-audited facilities |
| Affordability & Generic Access | Cost-competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Aceclofenac is a non-steroidal anti-inflammatory drug (NSAID) commonly prescribed for the treatment of pain and inflammation associated with osteoarthritis, rheumatoid arthritis, and ankylosing spondylitis.
While Aceclofenac is generally well-tolerated, some users may experience side effects such as dizziness or drowsiness. Therefore, it is advisable to avoid driving or operating heavy machinery until you know how the medication affects you. Patients should consult their healthcare provider if such symptoms occur.
Salius Pharma supplies Aceclofenac primarily as an Active Pharmaceutical Ingredient (API). However, finished dosage forms (such as tablets) can be provided on request to suit local market requirements.
To order Aceclofenac API, contact Salius Pharma with details such as the required quantity, destination country, applicable regulatory documentation (e.g., GMP, DMF), and packaging needs. We cater to regional compliance standards across Latin America.
Salius Pharma actively exports Aceclofenac to several Latin American countries, including Mexico, Colombia, Argentina, Chile, and Peru. All exports comply with respective national health authority requirements, ensuring quality and regulatory alignment.
Looking to source Aceclofenac or other high-quality pharmaceutical products? We’re here to help. Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support, our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.