
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
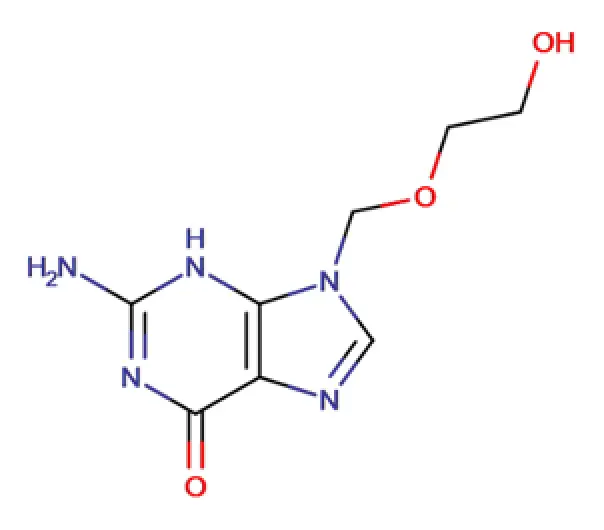
API
BP/USP
C8H11N5O3
2 amino 1,9 dihydro 9 ((2 hydroxyethoxy) methyl) 3H purin 6 one
59277-89-3
225.20 g/mol
Antiviral
Guanine (purine)
Nucleoside analogue
| Appearance | White to almost white crystalline powder |
|---|---|
| Solubility | Slightly soluble in water & ethanol; practically insoluble in chloroform & acetone |
| Melting point | 256–258 °C (decomposes) |
| pH | 10.5 – 11.5 |
Acyclovir is a synthetic acyclic guanine nucleoside analog that is selectively activated in herpesvirus-infected cells. It’s first phosphorylated by the viral thymidine kinase into acyclovir monophosphate and then converted by host kinases to the active acyclovir triphosphate. This metabolite competes with deoxyguanosine triphosphate for incorporation by viral DNA polymerase; lacking a 3′ OH group, its insertion causes premature chain termination and permanently deactivates the polymerase, effectively halting viral DNA replication with minimal effect on host cells.
Acyclovir is selectively activated in herpesvirus-infected cells via viral thymidine kinase, then converted to acyclovir triphosphate. This active form competitively inhibits viral DNA polymerase and, upon incorporation into viral DNA, causes irreversible chain termination, halting replication with minimal impact on host cells.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO-GMP, ISO 9001:2015, FDA-audited facilities |
| Affordability & Generic Access | Cost-competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
India has emerged as a major global supplier of antiviral APIs due to its strong manufacturing capabilities, pharma compliance, and competitive costs. Exporters like Salius Pharma deliver BP/USP/EP grade Acyclovir trusted by international formulation companies.
Acyclovir is available in various forms, including oral tablets, topical creams, and intravenous injections. The choice of administration depends on the type and severity of the infection.
Acyclovir API has strong export demand in LATAM, Asia-Pacific, Middle East, and African markets, driven by the growing need for affordable antiviral therapies and increasing incidences of herpes-related infections.
The global Acyclovir market is experiencing steady growth due to the increasing prevalence of herpes-related infections and the rising demand for effective antiviral treatments. The market is expected to expand further with the development of generic formulations and increased access in emerging markets.
Yes, Salius Pharma exports Acyclovir API. The company is a Government of India recognized Star Export House and is ISO 9001:2015 certified. They are involved in the export of various Active Pharmaceutical Ingredients (APIs) globally, including Acyclovir. Salius Pharma has a strong presence in international markets and is committed to providing high-quality pharmaceutical products worldwide.
Acyclovir API is typically packed in 25kg HDPE/Fibre drums with double-layer liners to prevent moisture ingress. Customized packaging can also be provided to meet importer regulatory requirements.
Looking to source Acyclovir or other high-quality pharmaceutical products? We’re here to help. Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support, our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.