
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
BP/USP/EP
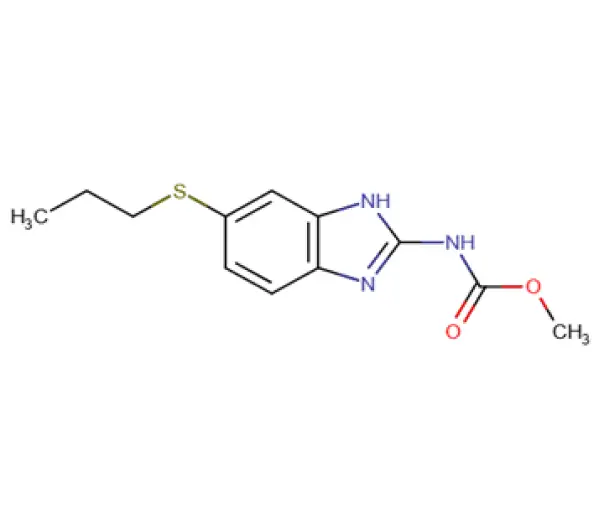
C12H15N3O2S
methyl N-[6-(propylsulfanyl)-1H-1,3-benzodiazol-2-yl]carbamate
54965-21-8
265.331 g/mol
Benzimidazole
Anthelminthic
| Appearance | White to slightly yellow crystalline powder |
|---|---|
| Solubility | Practically insoluble in water; slightly soluble in methanol and alcohol |
| Melting point | 208–210 °C |
| pH | 6.0–7.0 |
Albendazole is a benzimidazole anthelmintic used to treat parenchymal neurocysticercosis and other helminth infections. It is a broad-spectrum anthelmintic. The principal mode of action for albendazole is by its inhibitory effect on tubulin polymerization which results in the loss of cytoplasmic microtubules.
Albendazole causes degenerative alterations in the tegument and intestinal cells of the worm by diminishing its energy production, ultimately leading to immobilization and death of the parasite. It works by binding to the colchicine-sensitive site of tubulin, thus inhibiting its polymerization or assembly into microtubules.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO-GMP, ISO 9001:2015, FDA-audited facilities |
| Affordability & Generic Access | Cost-competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Albendazole is widely procured by governments and NGOs for mass deworming programs due to its effectiveness, low treatment cost, and inclusion in WHO-recommended parasitic disease control initiatives.
While many countries accept BP/USP/EP-compliant material, some importers may request additional documentation such as WHO-PQ support, CEP status, or local regulatory registration. Our team assists buyers based on their country-specific compliance needs.
Yes — stability studies under Zone III/IV conditions support markets like Africa, LATAM, Southeast Asia, and GCC, where high temperatures and humidity demand enhanced shelf-life assurance.
Stock availability allows rapid delivery, typically 2–3 weeks, depending on order size and destination. Dedicated logistics support ensures smooth customs clearance and timely supply.
Strict in-process controls, validated manufacturing procedures, and comprehensive analytical testing guarantee uniform impurity profiles and potency, supporting uninterrupted production for global formulators.
Secure export-grade HDPE drums with tamper-evident sealing ensure moisture protection and stability throughout sea and air shipment routes.
Looking to source Albendazole or other high-quality pharmaceutical products? We’re here to help. Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support, our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.