
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
USP/EP
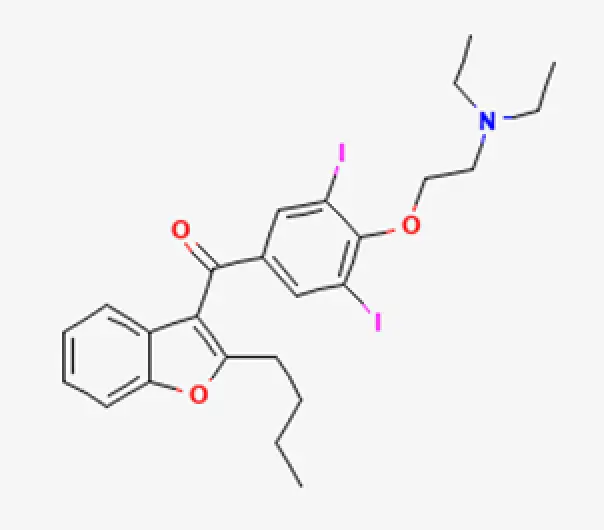
C25H30ClI2NO3
{2-[4-(2-butyl-1-benzofuran-3-carbonyl)-2,6-diiodophenoxy] ethyl}diethylamine hydrochloride
7177-48-2
681.773 g/mol
Aryl-phenylketones
Antiarrhythmics, Class III
| Appearance | White to off-white crystalline powder |
|---|---|
| Solubility | Sparingly soluble in water; soluble in alcohol, methanol, and chloroform |
| Melting point | 157–159 °C |
| pH | 4-6 |
Amiodarone is a benzofuran derivative, an anti-arrhythmic drug used commonly in a variety of settings. Most known for its approved indication in life-threatening ventricular arrhythmias, it is also used off-label in the outpatient and inpatient setting for atrial fibrillation. Because of its ability to cause serious toxicity and possibly death, amiodarone use should be reserved for its approved indications, according to prescribing information.
Amiodarone is considered a class III anti-arrhythmic drug. It blocks potassium currents that cause repolarization of the heart muscle during the third phase of the cardiac action potential. As a result, amiodarone increases the duration of the action potential as well as the effective refractory period for cardiac cells (myocytes). Therefore, cardiac muscle cell excitability is reduced, preventing and treating abnormal heart rhythms.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO-GMP, ISO 9001:2015, FDA-audited facilities |
| Affordability & Generic Access | Cost-competitive generics for global supply |
| Export Experience | Proven record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Looking to source Amiodarone Hydrochloride or other high-quality pharmaceutical products? We’re here to help. Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support, our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.