
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
BP/USP/EP
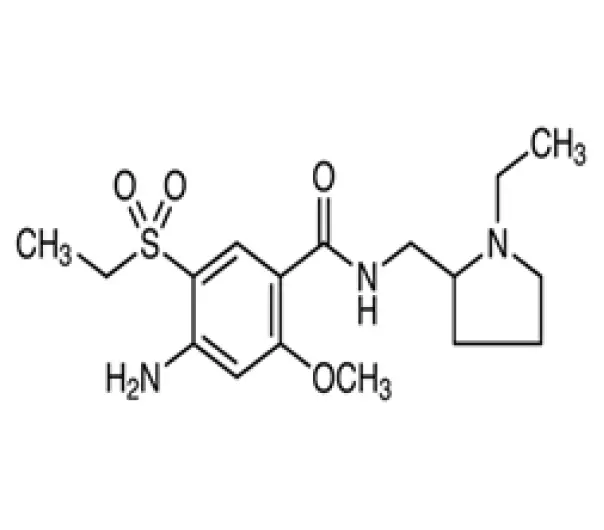
C₁₅H₂₁N₃O₄S
N-(1-methylpyrrolidin-2-yl)-5-amino-6-methoxy-4-(ethylsulfonyl)benzamide
71675-85-9
~339.42 g/mol
Atypical antipsychotic agent
| Appearance | White to off-white crystalline powder |
|---|---|
| Solubility | Slightly soluble in water; soluble in acidic solutions |
| Melting point | 176–178 °C |
| pH | 9.37 |
Amisulpride is an atypical antipsychotic used to treat schizophrenia and related disorders. It works by selectively blocking dopamine D2 and D3 receptors, helping to reduce symptoms like hallucinations and delusions. Supplied as a white to off-white crystalline powder, it meets BP, USP, and EP standards and is used as an API for making tablets and capsules. Amisulpride is known for effectively treating both positive and negative symptoms with fewer movement-related side effects.
Amisulpride works as a selective dopamine D2 and D3 receptor antagonist. By blocking these receptors in the brain, it helps normalize dopamine activity, which is often imbalanced in conditions like schizophrenia. This action reduces positive symptoms (such as hallucinations and delusions) and, at low doses, can also improve negative symptoms (such as social withdrawal and lack of motivation) without causing significant movement-related side effects common with older antipsychotics.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO-GMP, ISO 9001:2015, FDA-audited facilities |
| Affordability & Generic Access | Cost-competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Looking to source Amisulpride or other high-quality pharmaceutical products? We’re here to help. Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support, our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.