
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
BP/USP
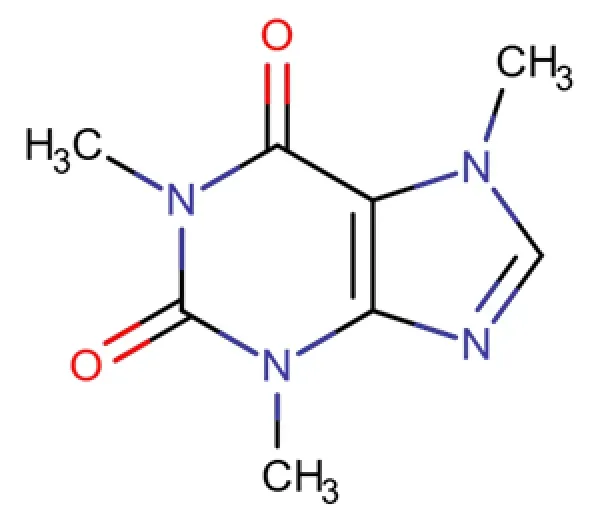
C8H10N4O2
1,3,7-trimethyl-2,6-dioxo-1,2,3,6-tetrahydro-7H-purine
58-08-2
194.1906 g/mol
Xanthines
Central Nervous System Stimulants
| Appearance | White, crystalline powder; odorless with a bitter taste |
|---|---|
| Solubility | In water: ~2.17 g/100 mL at 25°C (solubility increases with temperature), also soluble in: ethanol, chloroform, acetone & slightly soluble in: diethyl ether |
| Melting point | 234–236°C |
| pH | 6.9–7.3 |
Caffeine is a drug of the methylxanthine class used for a variety of purposes, including certain respiratory conditions of the premature newborn, pain relief, and combat drowsiness. Caffeine is similar in chemical structure to Theophylline and Theobromine.
Respiratory System:
Enhances respiratory drive by stimulating the brain’s response to CO₂ and increasing diaphragm contractility—used in treating apnea of prematurity.
Central Nervous System:
Blocks all four adenosine receptor subtypes (A1, A2a, A2b, A3); alertness effects are mainly due to A2a receptor antagonism.
Renal System:
Acts as a diuretic by increasing renal blood flow, glomerular filtration, and sodium excretion.
Cardiovascular System:
Increases heart activity via adenosine receptor blockade and catecholamine release. Causes both vasodilation (via nitric oxide) and vasoconstriction (via catecholamines), raising systolic blood pressure and relieving migraine by constricting dilated brain vessels.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO-GMP, ISO 9001:2015, FDA-audited facilities |
| Affordability & Generic Access | Cost-competitive generics for global supply |
| Export Experience | Proven record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Looking to source Caffeine or other high-quality pharmaceutical products? We’re here to help. Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support, our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.