
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
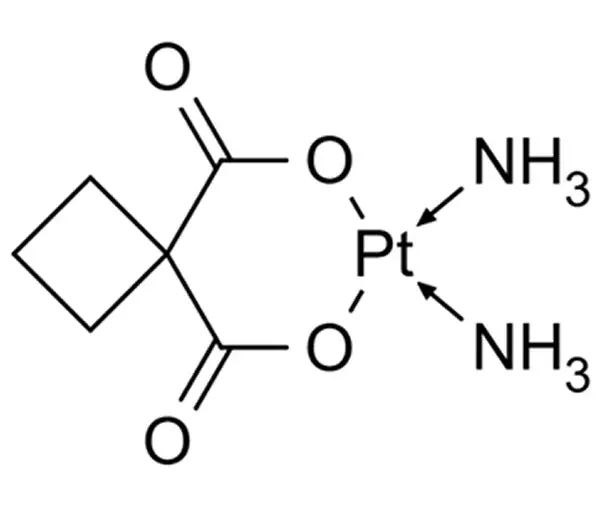
API
BP/USP
C6H12N2O4Pt
7,7-diamino-6,8-dioxa-7-platinaspiro[3.5]nonane-5,9-dione
41575-94-4
371.254 g/mol
Platinum Compounds
Antineoplastic
| Appearance | White to off-white crystalline powder |
|---|---|
| Solubility | Freely soluble in water, Slightly soluble in methanol, Practically insoluble in ethanol, acetone, and ether |
| Melting point | 253–255 °C |
| pH | 5.0 to 7.0 |
Carboplatin is an organoplatinum antineoplastic alkylating agent used in the treatment of advanced ovarian carcinoma. Early clinical studies of carboplatin were performed in 1982. Carboplatin was developed as an analog of cisplatin with reduced nephrotoxicity and vomiting. Carboplatin was granted FDA approval on 3 March 1989.
Carboplatin predominantly acts by attaching alkyl groups to nucleotides, leading to the formation of monoadducts, and DNA fragmenting when repair enzymes attempt to correct the error. 2% of carboplatin's activity comes from DNA cross-linking from a base on one strand to a base on another, preventing DNA strands from separating for synthesis or transcription. Finally, carboplatin can induce several different mutations.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO-GMP, ISO 9001:2015, FDA-audited facilities |
| Affordability & Generic Access | Cost-competitive generics for global supply |
| Export Experience | Proven record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Looking to source Carboplatin or other high-quality pharmaceutical products? We’re here to help. Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support, our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.