
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
BP/USP
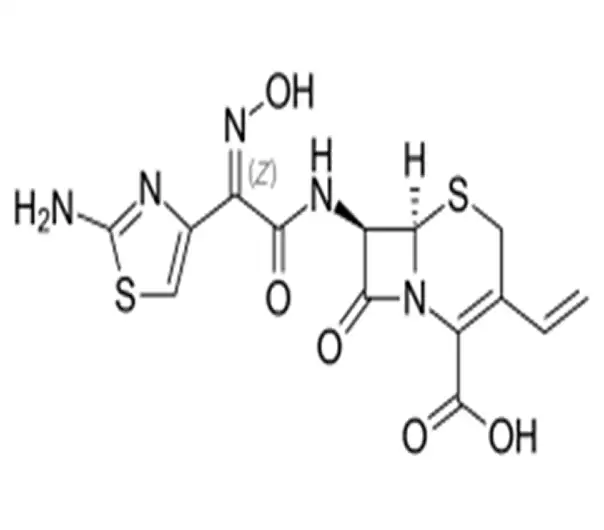
C14H13N5O5S2
(6R,7R)-7-[(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-(N-hydroxyimino)acetamido]-3-ethenyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid
91832-40-5
395.414 g/mol
Cephalosporins
Antibiotic- Cephalosporins
| Appearance | White to light yellowish crystalline powder |
|---|---|
| Solubility | Practically insoluble in water, slightly soluble in methanol, freely soluble in dimethylformamide (DMF), very slightly soluble in ethanol and acetone. |
| Melting point | 226–230 °C |
| pH | 226–230 °C |
Cefdinir, also known as Omnicef, is a semi-synthetic, broad-spectrum antibiotic belonging to the third generation of the cephalosporin class. It has been proven to be effective for the treatment of common bacterial infections in the ear, sinus, throat, lungs, and skin. Cefdinir was approved by the FDA in 1997 to treat a variety of mild to moderate infections and was initially marketed by AbbVie. Because of its chemical structure, it is effective against organisms that are resistant to first-line cephalosporin therapy due to the production of beta-lactamase enzymes.
With a mechanism like other beta-lactam antibiotics, the bactericidal activity of cefdinir is caused by the inhibition of cell wall synthesis via binding to penicillin-binding proteins (PBPs). Cefdinir, like other cephalosporins, penetrates the bacterial cell wall, combats inactivation by beta-lactamase enzymes, and inactivates penicillin-binding proteins. This interferes with the final step of transpeptidation in cell walls, eventually leading to cell lysis, which eventually leads to the death of bacteria that are susceptible to this drug.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO-GMP, ISO 9001:2015, FDA-audited facilities |
| Affordability & Generic Access | Cost-competitive generics for global supply |
| Export Experience | Proven record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Looking to source Cefdinir or other high-quality pharmaceutical products? We’re here to help. Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support, our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.