
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
BP/USP
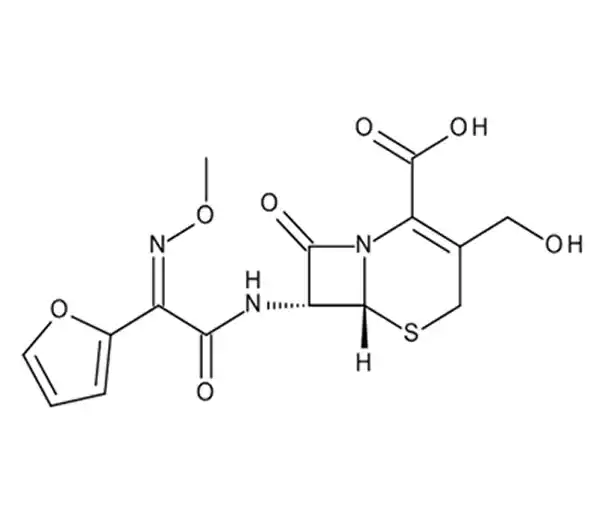
C20H22N4O10S
1-acetyloxyethyl (6R,7R)-3-(carbamoyloxymethyl)-7-[[(2Z)-2-(furan-2-yl)-2-methoxyiminoacetyl]amino]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate
64544-07-6
510.475 g/mol
Cephalosporins
Antibiotic- Cephalosporins
| Appearance | White to yellowish crystalline powder |
|---|---|
| Solubility | Practically insoluble in water, freely soluble in acetone and chloroform, slightly soluble in ethanol |
| Melting point | 105–110 °C |
| pH | 4.0 to 6.0 |
Cefuroxime is a broad-spectrum third-generation cephalosporin antibiotic. It has a very long half-life compared to other cephalosporins and is high penetrable into the meninges, eyes, and inner ear. Ceftriaxone has broader and stronger gram-negative coverage than first or second-generation cephalosporins, but worse activity against methicillin-susceptible S.aureus.
Cefuroxime, like the penicillins, is a beta-lactam antibiotic. By binding to specific penicillin-binding proteins (PBPs) located inside the bacterial cell wall, it inhibits the third and last stage of bacterial cell wall synthesis. Cell lysis is then mediated by bacterial cell wall autolytic enzymes such as autolysins; it is possible that cefuroxime interferes with an autolysin inhibitor.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO-GMP, ISO 9001:2015, FDA-audited facilities |
| Affordability & Generic Access | Cost-competitive generics for global supply |
| Export Experience | Proven record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Cefuroxime Axetil is commonly prescribed for the treatment of bacterial infections such as pneumonia, bronchitis, otitis media, urinary tract infections, skin and soft tissue infections, intra-abdominal infections, sepsis, and meningitis. It is widely used in respiratory, genitourinary, and dermatological infectious disease therapy.
Salius Pharma facilitates market entry by providing complete regulatory documentation, including Drug Master Files (DMFs), Certificates of Analysis (CoAs), and CTD/eCTD dossiers. These documents are tailored to comply with the specific requirements of regulatory authorities such as NAFDAC (Nigeria), INVIMA (Colombia), the Drug Administration of Vietnam, and the Egyptian Drug Authority (EDA).
Prolonged use of Cefuroxime should be carefully monitored due to the risk of antimicrobial resistance, disruption of the gut microbiota, and secondary infections like Clostridioides difficile-associated diarrhea. Long-term therapy should only be pursued under strict medical supervision and supported by clear clinical indications.
Cefuroxime exerts its antibacterial effect by inhibiting bacterial cell wall synthesis. It binds to penicillin-binding proteins (PBPs), disrupting the final stage of peptidoglycan cross-linking, which leads to cell lysis and death. Although classified as a second-generation cephalosporin, it demonstrates strong activity against many Gram-negative and select Gram-positive pathogens.
Common side effects of Cefuroxime include gastrointestinal symptoms such as diarrhea and nausea, as well as injection site reactions like pain or swelling. Skin rashes may also occur. Rare but serious adverse effects include hypersensitivity reactions (e.g., anaphylaxis), biliary complications (e.g., biliary sludge), and hematological changes such as eosinophilia or thrombocytosis.
Looking to source Cefuroxime Axetil or other high-quality pharmaceutical products? We’re here to help. Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support, our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.