
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
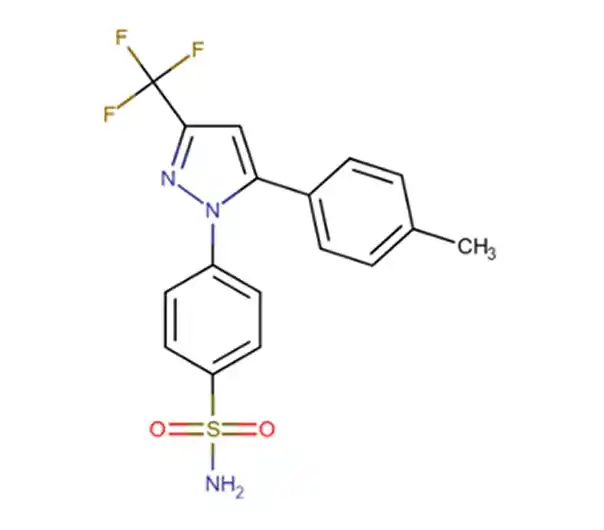
API
BP/USP
C17H14F3N3O2S
4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]benzene-1-sulfonamide
169590-42-5
381.372 g/mol
Phenylpyrazoles
Anti-Inflammatory Agents
| Appearance | White powder |
|---|---|
| Solubility | Freely soluble in water and ethanol, but practically insoluble in ether |
| Melting point | 237 to 243 °C |
| pH | 4.0 to 5.0 |
Celecoxib, a selective cyclooxygenase-2 (COX-2) inhibitor, is a nonsteroidal anti-inflammatory drug (NSAID) which is known for its decreased risk of causing gastrointestinal bleeding compared to other NSAIDS. It is used to manage symptoms of various types of arthritis pain and in familial adenomatous polyposis (FAP) to reduce precancerous polyps in the colon. It is marketed by Pfizer under the brand name Celebrex and was initially granted FDA approval in 1998. Interestingly, selective COX-2 inhibitors (especially celecoxib), have been evaluated as potential cancer chemo preventive and therapeutic drugs in clinical trials for a variety of malignancies.
Celecoxib is a selective noncompetitive inhibitor of cyclooxygenase-2 (COX-2) enzyme. COX-2 is expressed heavily in inflamed tissues where it is induced by inflammatory mediators. The inhibition of this enzyme reduces the synthesis of metabolites that include prostaglandin E2 (PGE2), prostacyclin (PGI2), thromboxane (TXA2), prostaglandin D2 (PGD2), and prostaglandin F2 (PGF2). By inhibiting prostaglandin synthesis, non-steroidal anti-inflammatory drugs (NSAIDs) cause mucosal damage, ulceration and ulcer complication throughout the gastrointestinal tract.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO-GMP, ISO 9001:2015, FDA-audited facilities |
| Affordability & Generic Access | Cost-competitive generics for global supply |
| Export Experience | Proven record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Celecoxib is most prescribed in rheumatology, orthopedics, and pain management. It is used for the treatment of osteoarthritis, rheumatoid arthritis, ankylosing spondylitis, acute pain, primary dysmenorrhea, and postoperative pain.
Salius Pharma facilitates market entry by providing regulatory support through well-prepared Drug Master Files (DMFs), Certificates of Analysis (CoAs), and CTD/eCTD dossiers. These are tailored to meet the submission requirements of regulatory agencies in South African countries.
Long-term use of Celecoxib should be approached with caution, particularly in patients with cardiovascular disease, hypertension, renal impairment, or gastrointestinal disorders. Though it has a lower risk of gastric ulceration compared to traditional NSAIDs, it may increase the risk of serious cardiovascular events, such as myocardial infarction or stroke, especially at high doses or with prolonged use.
Celecoxib is a selective cyclooxygenase-2 (COX-2) inhibitor. It works by inhibiting the COX-2 enzyme, which plays a key role in the synthesis of prostaglandins involved in inflammation, pain, and fever. By selectively targeting COX-2 while sparing COX-1, Celecoxib reduces inflammation and pain with a lower incidence of gastrointestinal side effects typical of non-selective NSAIDs.
Common side effects of Celecoxib include dyspepsia (indigestion), diarrhea, abdominal pain, and headache. Less common but serious adverse events may include hypertension, renal dysfunction, gastrointestinal bleeding, and cardiovascular events such as thromboembolism.
Looking to source Celecoxib or other high-quality pharmaceutical products? We’re here to help. Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support, our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.