
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
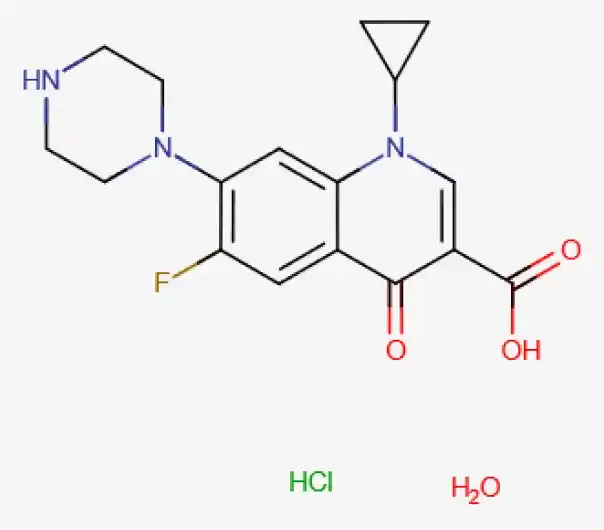
API
BP/USP
C17H21ClFN3O4
(Z)-but-2-enedioic acid;3-(4-chlorophenyl)-N,N-dimethyl-3-pyridin-2-ylpropan-1-amine
86393-32-0
385.82g/mol
Quinoline carboxylic acids
Anti-Bacterial Agents
| Appearance | White to slightly yellowish crystalline powder |
|---|---|
| Solubility | Freely soluble in water, slightly soluble in methanol and ethanol, practically insoluble in chloroform and ether |
| Melting point | 261–265 °C |
| pH | 3.0 to 4.5 |
Ciprofloxacin is a second-generation fluoroquinolone that has spawned many derivative antibiotics. It is formulated for oral, intravenous, intratympanic, ophthalmic, and optic administration for several bacterial infections.
Ciprofloxacin acts on bacterial topoisomerase II (DNA gyrase) and topoisomerase IV. Ciprofloxacin's targeting of the alpha subunits of DNA gyrase prevents it from supercoiling the bacterial DNA which prevents DNA replication.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO-GMP, ISO 9001:2015, FDA-audited facilities |
| Affordability & Generic Access | Cost-competitive generics for global supply |
| Export Experience | Proven record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Ciprofloxacin HCl is commonly used to treat bacterial infections such as urinary tract infections (UTIs), respiratory infections, gastrointestinal infections, skin and soft tissue infections, and bone and joint infections. It is also used for prophylaxis in anthrax exposure and certain sexually transmitted infections.
Salius Pharma supports market entry by providing comprehensive regulatory documentation such as Drug Master Files (DMFs), Certificates of Analysis (CoAs), and CTD/eCTD dossiers, tailored to meet the requirements of authorities like BPOM (Indonesia), FDA (Philippines), NPRA (Malaysia), HSA (Singapore), and Thai FDA.
Patients should be monitored for tendon inflammation, CNS effects (e.g. dizziness, seizures), and QT prolongation. Avoid use in children and pregnant women unless necessary. Concomitant use with antacids, dairy products, or iron supplements may reduce its absorption.
Ciprofloxacin HCl inhibits bacterial DNA gyrase and topoisomerase IV, enzymes required for DNA replication and transcription, leading to bacterial cell death. It is bactericidal and effective against a broad spectrum of pathogens.
Common side effects include nausea, diarrhea, headache, and dizziness. Rare but serious side effects include tendon rupture, peripheral neuropathy, photosensitivity, and hypersensitivity reactions.
Looking to source Ciprofloxacin HCl or other high-quality pharmaceutical products? We’re here to help. Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support, our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.