
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
BP/USP
C63H88CoN14O14P
cyano[(1R,2R,3R,4R,6Z,8S,11Z,13S,14S,16Z,18S,19S)-8,13,18-tris(2-carbamoylethyl)-3,14,19-tris(carbamoylmethyl)-4-(2-{[(2R)-2-{[(2R,3S,4R,5S)-5-(5,6-dimethyl-1H-1,3-benzodiazol-1-yl)-4-hydroxy-2-(hydroxymethyl)oxolan-3-yl phosphonato]oxy}propyl]carbamoyl}ethyl)-1,4,6,9,9,14,16,19-octamethyl-20,21,22,23-tetraazapentacyclo[15.2.1.1^{2,5}.1^{7,10}.1^{12,15}]tricosa-5(23),6,10(22),11,15(21),16-hexaen-20-yl]cobaltylium
68-19-9
1355.3652 g/mol
Cobalamin derivatives
Vitamin B Complex
| Appearance | WHITE TO SLIGHTLY YELLOWISH, CRYSTALLINE POWDER. |
|---|---|
| Solubility | Freely soluble in water, slightly soluble in ethanol, insoluble in acetone, chloroform, and ether |
| Melting point | 210°C to 230°C |
| pH | 4.5 to 5.5 |
Cyanocobalamin corrects vitamin B12 deficiency and improves the symptoms and laboratory abnormalities associated with pernicious anemia (megaloblastic indices, gastrointestinal lesions, and neurologic damage). This drug aids in growth, cell reproduction, hematopoiesis, nucleoprotein, and myelin synthesis. It also plays an important role in fat metabolism, carbohydrate metabolism, as well as protein synthesis. Cells that undergo rapid division (for example, epithelial cells, bone marrow, and myeloid cells) have a high demand for vitamin B12.
Vitamin B12 serves as a cofactor for methionine synthase and L-methylmalonyl-CoA mutase enzymes. Methionine synthase is essential for the synthesis of purines and pyrimidines that form DNA. L-methylmalonyl-CoA mutase converts L-methylmalonyl-CoA to succinyl-CoA in the degradation of propionate, an important reaction required for both fat and protein metabolism. It is a lack of vitamin B12 cofactor in the above reaction and the resulting accumulation of methylmalonyl CoA that is believed to be responsible for the neurological manifestations of B12 deficiency. Succinyl-CoA is also necessary for the synthesis of hemoglobin.
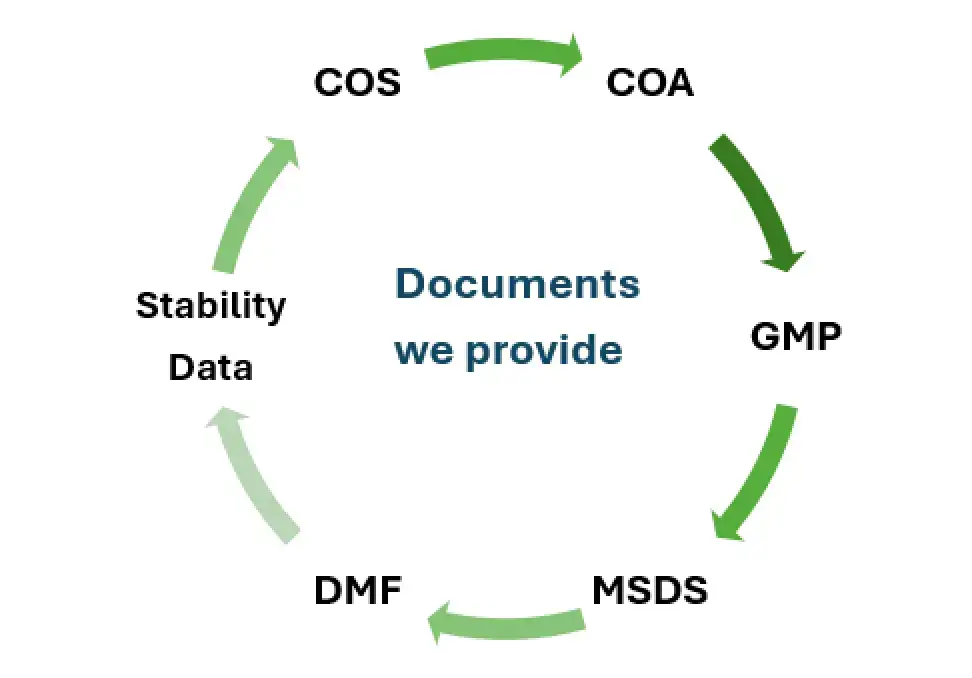
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Pharmaceuticals, nutraceuticals, injectables, oral supplements, fortified foods, and animal health sectors.
DMF/CoA/MSDS/GMP compliance certificates, stability data, and export documentation tailored to LATAM, EU, MENA & Asian markets.
Light-protected, airtight HDPE/Fiber drums, usually in 5–25 kg packs depending on buyer requirements.
Store at controlled ambient temperature, protect from light, and avoid moisture exposure to maintain potency.
Ensure pharmacopeial compliance (BP/USP), check regulatory filing requirements, permissible purity, and country-specific vitamin controls.
Assay ≥98%, low degradation impurities, stable polymorphic form, microbiological safety, and robust stability profile supporting dosage form manufacturing.
Looking to source Cyanocobalamin or other high-quality pharmaceutical products? We’re here to help. Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support, our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.