
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
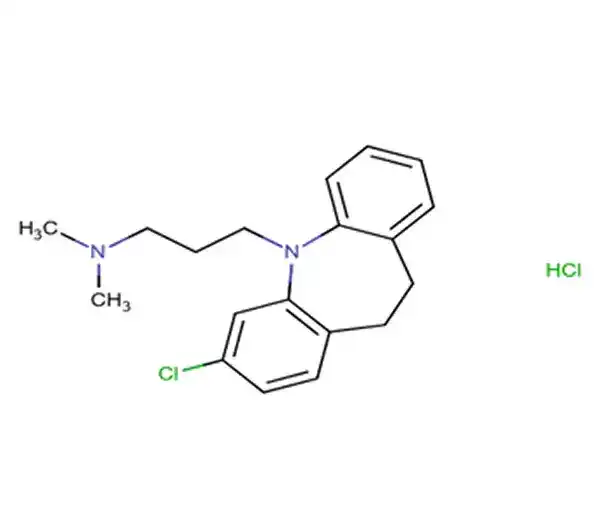
API
BP/USP
C21H23NO
dimethyl[(1S)-3-(naphthalen-1-yloxy)-1-phenylpropyl]amine
119356-77-3
305.4134 g/mol
Naphthyl derivatives
Selective Serotonin Reuptake Inhibitor (SSRI)
| Appearance | White to slightly yellowish, crystalline powder. |
|---|---|
| Solubility | Freely soluble in methanol, ethanol, and DMSO, sparingly soluble in water, practically insoluble in hexane and ether |
| Melting point | 175°C to 177°C |
| pH | 4.0 to 5.5 |
Dapoxetine, sold under the brand name Priligy among others, is a selective serotonin reuptake inhibitor (SSRI) used for the treatment of premature ejaculation (PE) in men ages 18 to 64 years old. Dapoxetine works by inhibiting the serotonin transporter, increasing serotonin's action at the postsynaptic cleft, and as a consequence promoting ejaculatory delay. As a member of the SSRI family, dapoxetine was initially created as an antidepressant.
The drug's mechanism of action is thought to be related to inhibition of neuronal reuptake of serotonin and subsequent potentiation of serotonin activity. The central ejaculatory neural circuit comprises spinal and cerebral areas that form a highly interconnected network. The sympathetic, parasympathetic, and somatic spinal centers, under the influence of sensory genital and cerebral stimuli integrated and processed at the spinal cord level, act in synergy to command physiologic events occurring during ejaculation. Experimental evidence indicates that serotonin (5-HT), throughout brain descending pathways, exerts an inhibitory role on ejaculation. To date, three 5-HT receptor subtypes (5-HT(1A), 5-HT(1B), and 5-HT(2C)) have been postulated to mediate 5-HT's modulating activity on ejaculation.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Men’s health and urology markets in regions with growing awareness and treatment adoption for premature ejaculation.
Regulatory documentation support (DMF/CoA/CTD), GMP manufacturing, flexible contract terms, responsive logistics, and shipment compliance for global markets.
HDPE/Fiber drum export-grade packaging, pharmacopeial purity compliance (BP/USP), controlled impurities, and validated stability data for finished dosage manufacturing.
It selectively inhibits serotonin reuptake to help increase ejaculatory control and improve patient outcomes.
Assay compliance to pharmacopeial limits, impurity profile, particle size consistency (for formulations), and validated stability data.
Yes — warnings related to dizziness, orthostatic hypotension, and interactions with other serotonergic drugs may need adaptation based on regulatory requirements.
Looking to source Dapoxetine or other high-quality pharmaceutical products? We’re here to help. Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support, our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.