
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
BP / USP / EP
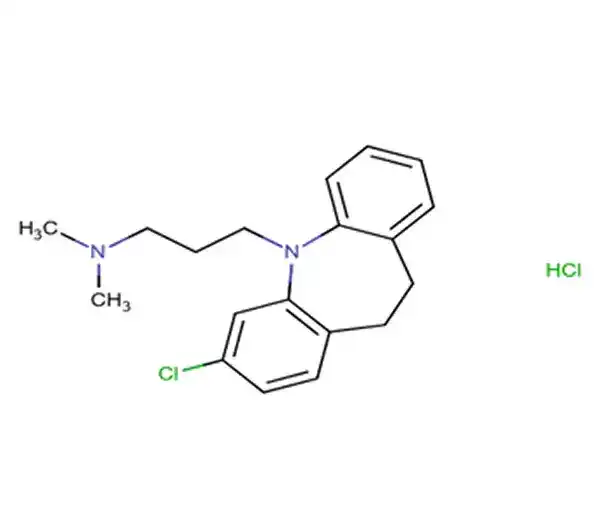
C₂₂H₂₉FO₅
(8S,9R,10S,11S,13S,14S,16R,17R)-9-Fluoro-11,17-dihydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]phenanthren-3-one
50-02-2
392.46 g/mol
Glucocorticoids (Corticosteroids)
Anti-inflammatory, Immunosuppressant, Antiallergic
| Appearance | White to slightly yellowish, crystalline powder. |
|---|---|
| Solubility | Practically insoluble in water, soluble in methanol and ethanol, slightly soluble in chloroform |
| Melting point | 134°C to 136°C |
| pH | 4.0 to 6.0 |
Corticosteroids exert their effects by binding to glucocorticoid receptors, where they suppress pro-inflammatory pathways and enhance anti-inflammatory responses. The duration of action of dexamethasone varies based on the route of administration. These drugs have a broad therapeutic window, as patients often require doses significantly higher than the body's natural hormone levels. It is important to counsel patients on the risks of hypothalamic-pituitary-adrenal (HPA) axis suppression and increased vulnerability to infections during corticosteroid therapy.
The short-term effects of corticosteroids are decreased vasodilation and permeability of capillaries, as well as decreased leukocyte migration to sites of inflammation. Corticosteroids binding to the glucocorticoid receptor mediates changes in gene expression that lead to multiple downstream effects over hours to days.
Glucocorticoids inhibit neutrophil apoptosis and demargination; they inhibit phospholipase A2, which decreases the formation of arachidonic acid derivatives; they inhibit NF-Kappa B and other inflammatory transcription factors; they promote anti-inflammatory genes like interleukin-10.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost-competitive generics for global supply |
| Export Experience | Proven record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Salius Pharma offers Dexamethasone API in 25 kg HDPE drums with double-layer polyethylene liners. Packaging complies with international transport and GMP standards, and custom packaging is available upon request.
The standard MOQ for Dexamethasone API is typically 1–5 kg due to its potency and low-dose usage. Flexible quantities can be arranged based on regulatory or commercial needs.
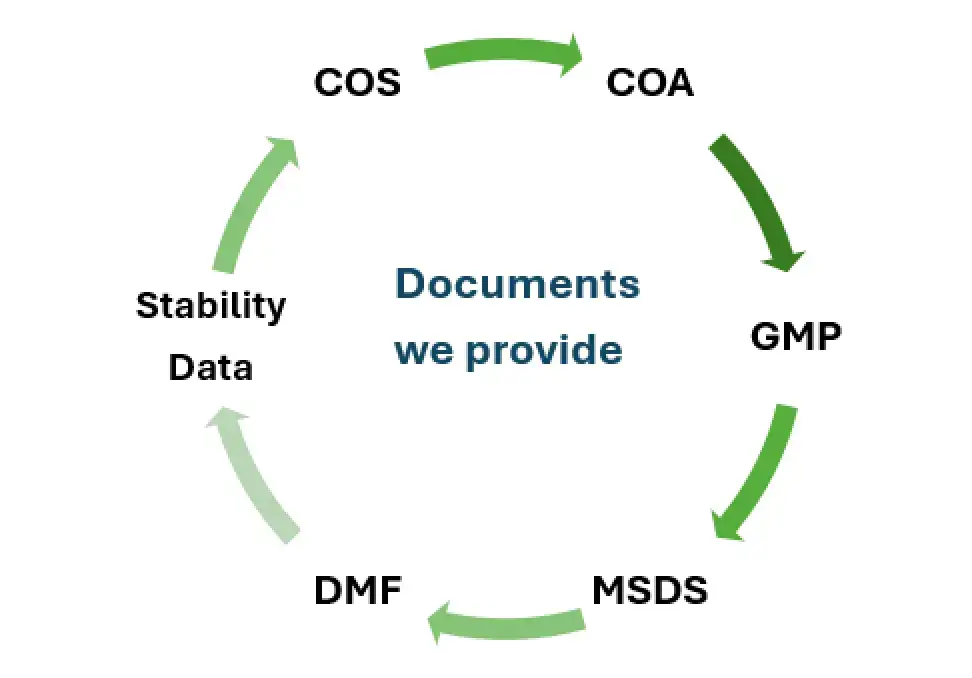
Each shipment includes a Certificate of Analysis (CoA), Certificate of Origin, Material Safety Data Sheet (MSDS), Packing List, and, if needed, a Certificate of Formula (CoF) or Free Sale Certificate. All documents are prepared to meet import-speaking country regulatory requirements.
Salius Pharma provides comprehensive support including DMFs (Drug Master Files), CTD/eCTD dossiers, stability data, and other technical documentation. The team also assists with country-specific submissions across regions like Africa, LATAM, CIS, and Southeast Asia.
Yes, Salius Pharma is a trusted exporter of Dexamethasone, supplying both regulated and semi-regulated markets across the globe. The company is distinguished by its regulatory expertise, consistent product quality, and streamlined international distribution capabilities.
Looking to source Dexamethasone or other high-quality pharmaceutical products? We’re here to help. Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support, our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.