
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
BP/EP/USP
C14H10Cl2NNaO2
sodium 2-[(2,6-dichlorophenyl)amino]phenylacetate
15307-79-6
318.13
Phenylacetic acid derivative
Anti-Inflammatory Agents (NSAIDs)
| Appearance | Off-White Crystalline Solid |
|---|---|
| Solubility | soluble in water |
| Melting point | 288-290°C |
| pH | 7.5 – 8.5 |
Diclofenac Sodium is the sodium salt form of diclofenac, a benzene acetic acid derivate and nonsteroidal anti-inflammatory drug (NSAID) with analgesic, antipyretic and anti-inflammatory activity. Diclofenac sodium is a non-selective reversible and competitive inhibitor of cyclooxygenase (COX), subsequently blocking the conversion of arachidonic acid into prostaglandin precursors. This leads to an inhibition of the formation of prostaglandins that are involved in pain, inflammation and fever.
Diclofenac sodium is a non-steroidal anti-inflammatory drug (NSAID) that works by inhibiting cyclooxygenase (COX) enzymes, which are responsible for producing prostaglandins—chemicals that cause inflammation, pain, and fever. By blocking COX enzymes, diclofenac reduces the production of prostaglandins, thereby decreasing inflammation and alleviating pain and fever. Its relatively higher selectivity for COX-2 makes it effective in treating inflammatory conditions such as arthritis and musculoskeletal pain.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Yes. Salius Pharma supplies Diclofenac Sodium API to regulated and semi-regulated international markets, supporting pharmaceutical manufacturers, importers, and formulators worldwide.
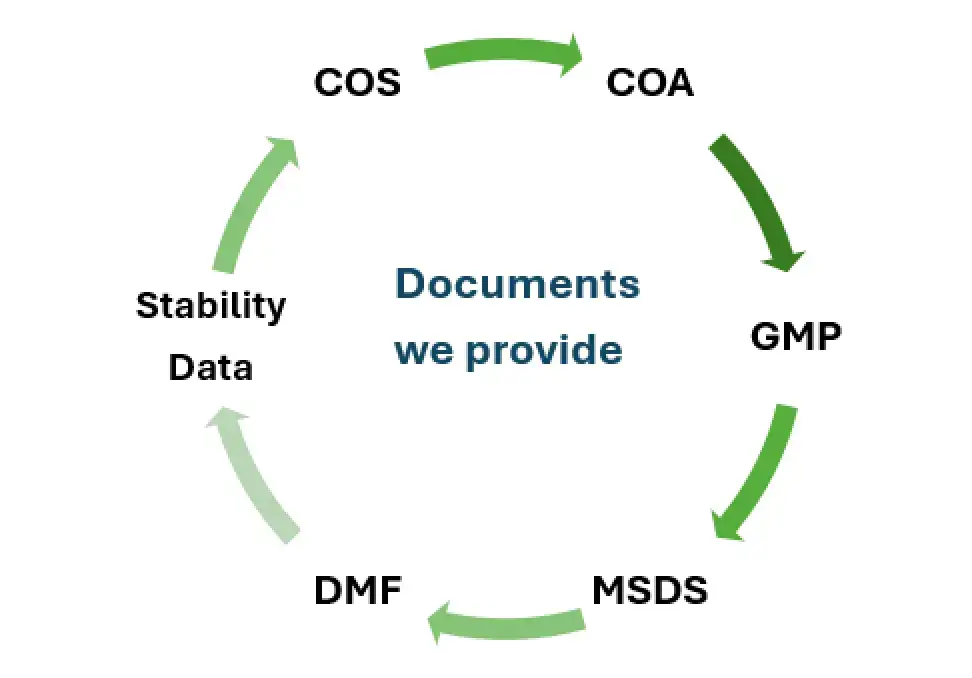
We provide DMF support (where applicable), COA, MSDS, TSE/BSE certificates, stability data, and full regulatory compliance documentation depending on market access needs.
We provide a complete regulatory support dossier depending on customer requirements, which may include: COA | MSDS | Stability data | TSE/BSE certificate | GMP-related documentation | DMF support (where applicable)
Export-grade, moisture-protected packing options include HDPE / Fibre drums with double poly-liners. Customized packing and labeling are available to meet country-specific regulatory norms.
Lead time generally ranges 2–4 weeks depending on order size and documentation. MOQ is usually 25 kg, with flexibility for strategic partners and large-volume purchasers.
Our Diclofenac Sodium API is suitable for tablets, capsules, topical gels/ointments, and injectable formulations, supporting customers in pain management and anti-inflammatory therapeutic categories.
Looking to source Diclofenac Sodium or other high-quality pharmaceutical products? We’re here to help. Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support, our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.