
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
BP/USP
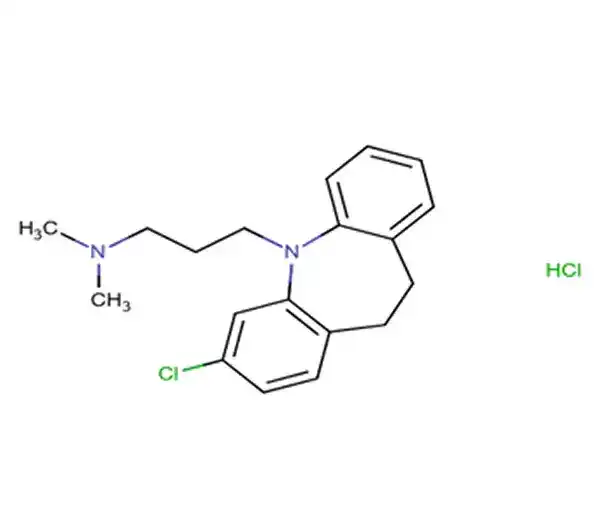
C5H10N2O7P2
(1-hydroxy-2-(1H-imidazol-1-yl)ethylidene)bis(phosphonic acid)
165800-06-6
272.09 g/mol
Bisphosphonate
Nitrogen-containing Bisphosphonate (third-generation bisphosphonate)
Anti-resorptive
| Appearance | White to off-white crystalline powder |
|---|---|
| Solubility | Freely soluble in water; practically insoluble in organic solvents |
| Melting point | ~ 235°C (decomposes) |
| pH | 2.0 – 3.5 |
Zoledronic Acid is a third-generation nitrogen-containing bisphosphonate used primarily for the treatment of bone complications such as osteoporosis, hypercalcemia of malignancy, Paget’s disease, and metastasis-related bone disorders.
Zoledronic Acid inhibits osteoclast-mediated bone resorption by binding strongly to bone mineral and suppressing farnesyl pyrophosphate synthase, a key enzyme in the mevalonate pathway.
This helps maintain bone density, reduces fractures, and lowers high calcium levels in cancer patients.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Zoledronic Acid is used to treat bone-related conditions such as osteoporosis, Paget’s disease, bone metastasis, and hypercalcemia caused by cancer.
It inhibits osteoclast-mediated bone resorption, helping strengthen bones, maintain bone density, and reduce fractures.
Salius Pharma supplies Zoledronic Acid as an Active Pharmaceutical Ingredient (API).
To order Zoledronic Acid API, you typically contact Salius Pharma. You’ll need to provide details such as desired quantity, intended market, regulatory requirements (e.g., DMF or GMP certificates), and packaging preferences.
Salius Pharma exports Zoledronic Acid to various countries worldwide, including regions such as Asia, Europe, the Middle East, Africa, North America & South America.
Looking to source Zoledronic Acid or other high-quality pharmaceutical products? We’re here to help. Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support, our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.